Yeast Western Blots
chemical protein extraction via hot hydroxide-SDS
derived from protocol described in Optimized Protein Extraction for Quantitative Proteomics of Yeasts by Tobias von der Haar
this modification by jtourig / zentlab, current as of Apr 2021
Intro
I’ve never had much luck with bead beating to extract yeast protein; it was always inefficient even with extended shaking, and I found it cumbersome overall. Seeking an alternative, I found this chemical extraction method from the proteomics paper cited above, as outlined in the left branch of the paper’s Figure 1 below. It relies on a short, hot hydroxide + SDS treatment to hydrolyze the cell wall and lyse the cells / solubilize protein…
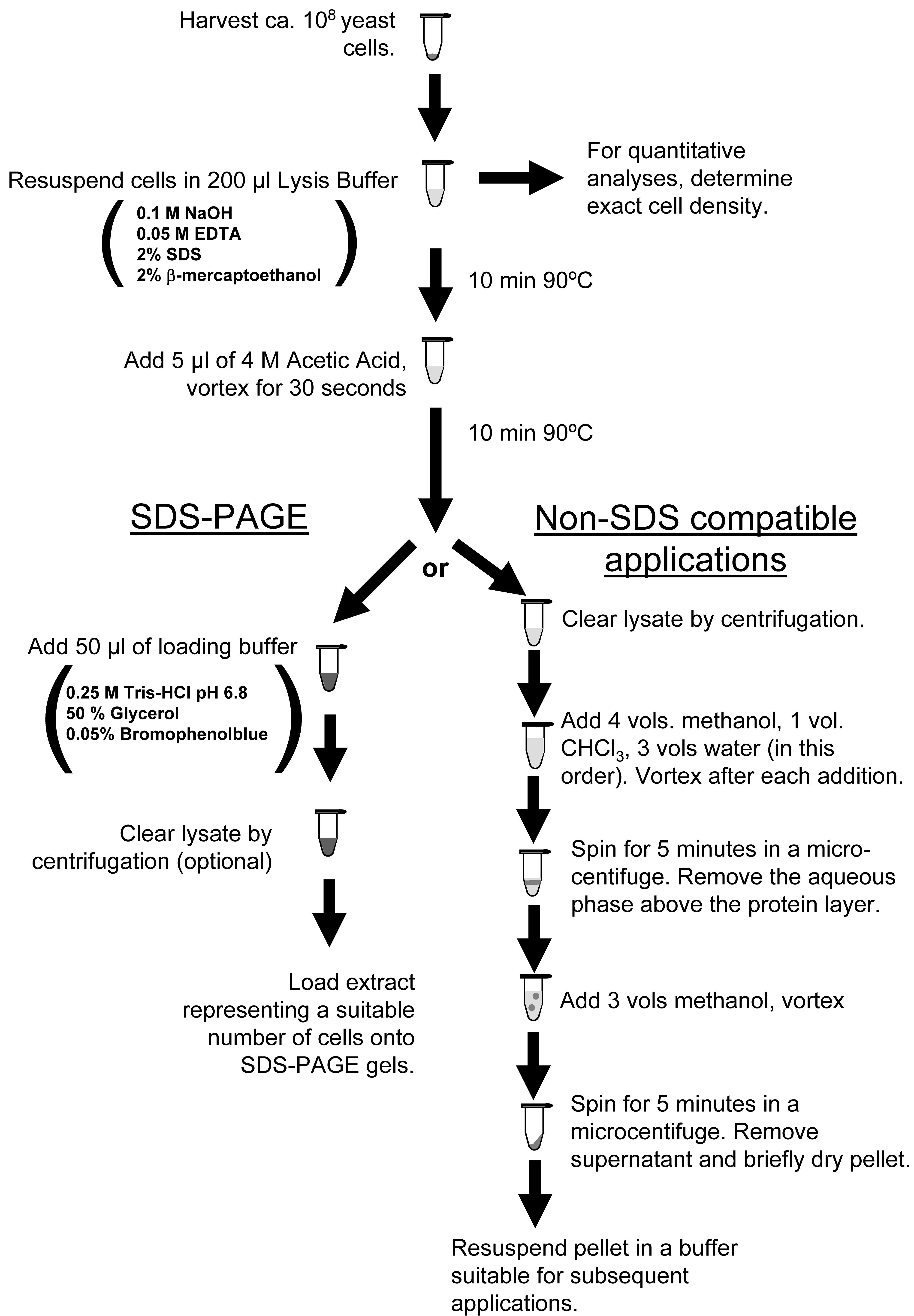 Fig 1 von der Haar POne 2007
Fig 1 von der Haar POne 2007
…with a few small modifications for time and volume.
Materials
You will need:
- NaOH-SDS Lysis Buffer (0.1M NaOH, 0.05M EDTA, 2% SDS)
- omit the stinky 2% BME reducing agent in the image - we add DTT later in the sample buffer
- 4M Acetic Acid (typically diluted from glacial acetic acid, which is ~17.4M)
- 4X LDS Sample Buffer (Bio-Rad #1610747)
- or whatever sample/loading buffer you’d like (adjust volumes as necessary)
- 1M DTT
- Yeast Cells (1-2ml of saturated overnight culture or ~6+ OD·ml of timecourse usually yields plenty)
Method
- Make sure you have a heat block turned on @ 95°C
- Pellet, aspirate, and resuspend cells in 150µl Lysis Buffer
- Heat @ 95°C for 5m
- Remove from heat and neutralize with 3.75µl 4M Acetic Acid, vortex
- this is important - if your sample buffer below turns yellow or clear, you forgot!
- Add 50µl 4X Sample Buffer (w/ DTT added 1:9, 100mM), vortex
- Heat @ 95°C for another 2m (you can set up your gel rig during this time)
- CLEAR THE LYSATE - spin for 2m at maximum speed to pellet cell debris (room temp)
- IMPORTANT - if your lanes are ever smeary, you might be dragging along DNA / insoluble gunk during electrophoresis
- Load the supernatant / soluble protein fraction in the gel relative to any normalization measurements made, and proceed with the blot.
Other Yeast Western Tips
- Make sure to check your loading and transfer, with Ponceau stain
- Most good yeast primaries work at 1:1000, and can be reused
- Use AdvanBlock-Fluor or -Chemi as blocking reagent and in primary dilution to improve sensitivity
- I find milk tends to lower signal of yeast westerns severely
- I usually probe for primary overnight @ 4°C, and wash it once quickly with PBS, then 3x with PBS + 0.1% Tween-20, for 5m, 6m, 7m (or just 3x over ~20m)
- Secondaries can be used at 1:10,000 for fluorescent and 1:20-30,000+ for HRP-conjugated antibodies
- liberally diluting the HRP secondary antibodies can help signal:noise a lot
- Use the LiCor (fluorescent blot) when you can - it’s convenient with great dynamic range
- Sometimes you must use chemiluminescence for sensitivity (at least with our equipment)
- image via a digital camera dock if you must use HRP (easy to make figures with software, good dynamic range)
- avoid film whenever possible (cumbersome and poor dynamic range)